
by manovermachine | Nov 16, 2022 | Philips Recalled BiPAP, CPAP, Ventilators
News from the FDA, DOJ and the stock market
November 16, 2022 – This week The New York Times published an article by investigative journalist Christina Jewett with some updates on the status of the Philips recall and the various lawsuits that are pending.
To review, the problems with their various breathing devices became apparent by 2015 and Philips Respironics didn’t do anything at the time. According to the Food and Drug Administration (FDA), years went by before the “company made cursory efforts to examine the problem.”
In April of 2021, the company claimed that it “realized the flaking foam contained potentially cancer-causing particles, setting off the largest and most disruptive medical device recall in more than a decade.” Since then, the recall of nearly fifteen million devices worldwide has caused patients to endure long waits for a replacement device. Some patients are receiving “refurbished” devices which they are reluctant to use.
“The F.D.A. shares the frustrations expressed by patients who are awaiting a resolution for this recall,” Dr. Jeff Shuren, director of the F.D.A.’s device center, said in a statement. “We have employed rarely used regulatory tools to hold Philips accountable and we will continue to communicate with the firm to assure they take appropriate steps to correct the product.”
The DOJ and Philips in Negotiations
The U.S. Justice Department is now negotiating the terms of a consent decree with Philips, “underscoring the deep concern about what the company knew – or should have known – before millions of people received devices that many believe caused devastating illnesses.”
Roy Jakobs, chief business leader for Philips’ Connected Care unit, told investors this past July that “We are in confidential discussions. We cannot disclose yet what the terms are and what impact they will have, but we understand that you are very much interested in that and the moment we can share we will share more details around this.”
Financial Impact and Layoffs
Fallout from the recall has resulted in the market value and stock plummeting by 70 percent and the company has been forced to lay off thousands of employees.
More than 69,000 complaints pertaining to cancer, difficulty breathing, and chest pain are being reviewed. There are 168 reported deaths, although experts say it may be difficult to determine whether the device caused any singular illness or death.
Finding and contacting patients who have these devices has been hampered by poor record-keeping and the industry-wide absence of a device tracking system.
Investigations Continue
The FDA has done extensive document reviews and has inspected the Philips Respironics facility in Murrysville, Pennsylvania. They found that by 2015, the company had learned that the foam in the devices was degrading. Emails and test reports showed that the foam could break down in as little as a year.
Philips finally began a formal internal investigation in 2019, and issued the recall in June 2021. By December 2021, their hazard assessment concluded that the device fumes presented a moderate risk for injury, rather than a serious risk. The FDA, however, considered their test methods flawed. Steve Klink, Philips spokesman, has said “At this stage, we can only apologize for the concern that has arisen, and we are working really hard to get to the bottom of the actual health risks.”

by manovermachine | Aug 23, 2022 | Philips Recalled BiPAP, CPAP, Ventilators
CEO steps down, additional devices added to recall
In a press release on August 16, 2022, Royal Philips announced that it is replacing its CEO Frans van Houten, who has been in the role for more than a decade, with Roy Jakobs. After the recall last year, revenues began slipping and fell 13 percent in second quarter 2022 as compared to second quarter 2021. The change in leadership will become official October 15, and Mr. van Houten will act as an adviser until April 30, 2023.
Following the recall of millions of ventilators in June 2021 that have been tied to 168 deaths, the company initiated a separate recall of additional devices that were not part of the original recall. All of its V60, V60 Plus, and V680 ventilators were recalled, more than 56,000 of which were distributed in the U.S. between May 2009 and December 2021, as reported by Fierce Biotech. All of these models are indicated for use only in healthcare facilities.
This recall was prompted by an issue with the internal power mechanism in the machines. An energy fluctuation could force the backup alarm controller to reboot, which could cause a complete shutdown of the device with no visible or audible alarm or warning.
Philips has issued guidance for healthcare providers who continue using the devices after taking some mitigating actions. They are strongly recommending that all users connect the devices to a nurse call or remote alarm system and make sure to respond promptly to every alarm issued by the device or the backup alarm system, regardless of priority level. They have also recommended that users install an oxygen analyzer and use pulse oximeters to serve as additional monitors for potential shutdowns.
Philips also recommends having backup ventilators at the ready in the event one of their devices does shut down. That would make it easier to immediately disconnect the patient from the faulty device and get them connected to a backup device as quickly as possible.
If these suggested additional protections are not instituted, Philips suggests that users analyze whether continuing to use the devices is worth the potential risk.
by manovermachine | Aug 18, 2022 | Philips Recalled BiPAP, CPAP, Ventilators
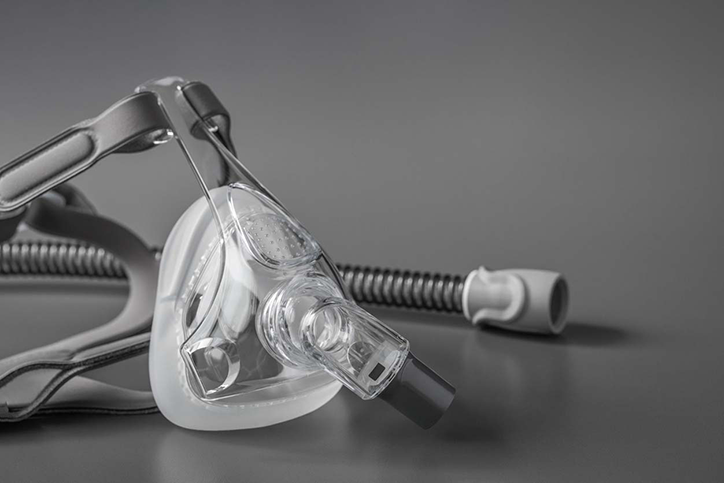
Bilevel Positive Airway Pressure (BiPAP) Machines
The Philips BiPAP device pushes air into your lungs through a mask or nasal plugs that are connected to a ventilator. Patients with chronic obstructive pulmonary disorder (COPD), obstructive sleep apnea, obesity hypoventilation syndrome, pneumonia, asthma flare-up, post-operative breathing difficulties, or neurological disease that disturbs breathing would be candidates for using a BiPAP. Patients with very poor breathing, reduced consciousness, or problems swallowing might not be helped by using a BiPAP.
Continuous Positive Airway Pressure (CPAP) Machines
Philips CPAP devices use mild air pressure from a small, sophisticated air compressor to keep airways open while sleeping. Patients with sleep-related breathing disorders are commonly treated with CPAP machines.
Ventilators
A Philips ventilator is a machine that moves air in and out of the lungs, doing the breathing work that the diaphragm and other muscles normally perform. Ventilators are for patients who are no longer able to breathe normally on their own.
Philips Respironics (Philips) is the maker of multiple types of breathing devices and supplies including BiPAP machines, CPAP machines, and ventilators. In June of 2021, Philips recalled several specific models of these devices, the majority of which are first-generation DreamStation products sold prior to April 2021. This recall involves millions of devices that may be affected by a defect that can harm the user.
These devices use a polyester-based polyurethane (PE-PUR) to lessen sound and vibration. This PE-PUR material can break down and pieces of black foam or certain chemicals could be breathed in or swallowed, potentially resulting in serious injury. The breakdown of this PE-PUR foam may be caused by hot and humid conditions or by the use of ozone cleaners or other cleaning methods not recommended by the manufacturer.
Injuries Due to the Recalled Philips Devices
Respiratory:
- Lung damage
- New or worsening asthma
- Pneumonia
- Respiratory failure (such as Acute Respiratory Distress Syndrome (ARDS))
- Pleural effusion
- Reactive Airway Disease (RAD)
Cancer:
- Blood, Lymph Node, and Oral Cancers:
- Acute Myeloid Leukemia (AML)
- Blood Cancer
- Bone Marrow Cancer
- Esophageal Cancer
- Hematopoietic Cancer
- Laryngeal Cancer
- Leukemia
- Lymphoma
- Multiple Myeloma
- Nasal Cancer
- Non-Hodgkin’s Lymphoma
- Soft Palate Cancer
- Sinus Cancer
- Throat Cancer
- Tonsil Cancer
- Thyroid Cancers:
- Thyroid Cancer
- Papillary Cancer
- Other Cancers:
- Kidney Cancer
- Liver Cancer
- Lung Cancer
- Bladder Cancer
Other:
- Severe ear, eye, nose, throat, sinus, oral cavity inflammation and injury including nodules, cysts, and tumors
- Sarcoidosis (particularly of the lungs and/or lymph nodes) that required treatment
- Kidney damage (acute kidney injury or chronic kidney disease)
- Liver damage (acute liver failure or chronic liver disease)
FDA Recommendations
The FDA has issued recommendations for patients and their caregivers who use recalled ventilators at home, and recommendations for health care providers and facilities. Separate recommendations were issued to patients and their caregivers who use the recalled BiPAP or recalled CPAP devices.
See the list of recalled devices.

by manovermachine | Aug 17, 2022 | Philips Recalled BiPAP, CPAP, Ventilators
In a press release on August 16, 2022, Royal Philips announced that it is replacing its CEO Frans van Houten, who has been in the role for more than a decade, with Roy Jakobs. After the recall last year, revenues began slipping and fell 13 percent in second quarter 2022 as compared to second quarter 2021. The change in leadership will become official October 15, and Mr. van Houten will act as an adviser until April 30, 2023.
Following the recall of millions of ventilators in June 2021 that have been tied to 168 deaths, the company initiated a separate recall of additional devices that were not part of the original recall. All of its V60, V60 Plus, and V680 ventilators were recalled, more than 56,000 of which were distributed in the U.S. between May 2009 and December 2021, as reported by Fierce Biotech. All of these models are indicated for use only in healthcare facilities.
This recall was prompted by an issue with the internal power mechanism in the machines. An energy fluctuation could force the backup alarm controller to reboot, which could cause a complete shutdown of the device with no visible or audible alarm or warning.
Philips has issued guidance for healthcare providers who continue using the devices after taking some mitigating actions. They are strongly recommending that all users connect the devices to a nurse call or remote alarm system and make sure to respond promptly to every alarm issued by the device or the backup alarm system, regardless of priority level. They have also recommended that users install an oxygen analyzer and use pulse oximeters to serve as additional monitors for potential shutdowns.
Philips also recommends having backup ventilators at the ready in the event one of their devices does shut down. That would make it easier to immediately disconnect the patient from the faulty device and get them connected to a backup device as quickly as possible.


