by manovermachine | Jan 26, 2023 | Zantac Linked Cancers
Zantac Lawsuits in New York Consolidated
More than 40 Zantac lawsuits pending in New York have been consolidated by New York’s Litigation Coordinating Panel on January 26. The plaintiffs in the lead case of Samuel v. Boehringer Ingelheim Pharmaceuticals Inc. et al filed a motion for coordination in October 2022, and in November the defendants filed their response, stating they do not oppose coordination of pretrial proceedings. Attorneys representing Samuel and other plaintiffs released a statement saying “We are pleased that there will be a mass litigation coordinated in New York to pursue our clients’ Zantac claims in light of the unfortunate MDL court’s actions. This is a welcome alternative.”
Jason T. Brown of Brown LLC, who represents other plaintiffs in the now-consolidated action, said Thursday, “We look forward to having the opportunity to proceed with representing alleged cancer victims of Zantac in the consolidated litigation so they can have their day in court in New York.”
++++++++++
Federal Lawsuits Against Zantac Dismissed by Judge, Lawsuits filed in state courts nationwide will go to trial in 2023
After a federal judge’s controversial decision to dismiss all federal Zantac lawsuits, plaintiffs have joined together to file their claims in Delaware state courts, where the lawsuits remain viable. The Multidistrict Litigation (MDL) judge excluded all of the plaintiffs’ expert witnesses and made his decision after finding that there was insufficient scientific evidence to establish that the recalled heartburn drug causes cancer under federal court system standards. As a result, all Zantac lawsuits pending in federal courts will be dismissed. Plaintiffs will likely appeal this decision. If the MDL judge’s decision to exclude plaintiffs’ expert testimony is reversed, claims may be reinstated and returned to the trial court.
State Court Zantac Lawsuits Continue
A complaint was filed last month in the Superior Court of the State of Delaware on behalf of 30 individuals against various makers of the drug, including GlaxoSmithKline, Pfizer, Boehringer Ingelheim, Sanofi-Aventis, Patheon Manufacturing Services, and DSM Pharmaceuticals, Inc.
The lawsuit states “Defendants were fully aware of the safety risks of Zantac, particularly the carcinogenic potential of Zantac as it transforms into NDMA within the chemical environment of the human body. Nonetheless, Defendants deliberately crafted their label, marketing, and promotion to mislead consumers.”
There are still approximately 50,000 Zantac lawsuits still pending in state courts nationwide. One bladder cancer lawsuit filed by James Goetz was expected to start in February 2023, however a settlement has been reached to resolve the case.
California state court Zantac trials are scheduled to go before juries in May, August, and October this year. Successful lawsuits will put pressure on the drug companies to negotiate settlements.
by manovermachine | Dec 16, 2022 | Camp Lejeune Contaminated Water
States step up to combat PFAS
Nearly all Americans are exposed to water tainted with low levels of toxic chemicals. The Legal Examiner recently stated that 97% of the US population has these chemicals in their bloodstream. Perfluoroalkyl and polyfluoroalkyl substances (PFAS) are also called “forever chemicals” because they don’t break down in the environment.
PFAS have been present in drinking water since the 1940s and a recent study found that the contamination of soil, rain, and surface water is so pervasive that it cannot be reversed without advanced or expensive technology. More than 2,800 sites in all 50 states are contaminated by PFAS.
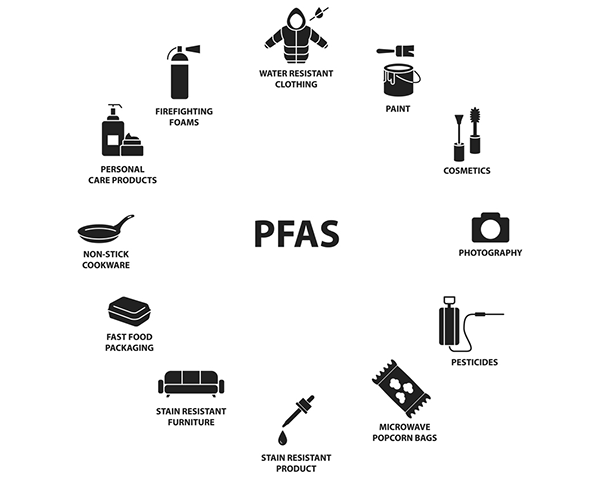
What Are PFAS Used For?
PFAS are used for nonstick pans, waterproofing outdoor clothing, food packaging, carpeting, and firefighting foam used on military bases and commercial airports. They are also found in waterproof cosmetics, sunscreen, shampoo, and shaving cream. Waxes, paints, and stains also contain PFAS. They are literally present in abundance in everyday life.
What Are the Health Risks From PFAS?
Research has linked PFAS to a host of health problems. Kidney cancer, testicular cancer, endocrine disruption and even an increased risk of contracting infectious diseases like COVID-19 as well as reducing the effectiveness of vaccines.
The toxic contamination of water at more than 400 active and closed military bases has drawn a lot of attention due to the devastating illnesses and adverse health effects. The most infamous case involves North Carolina’s Camp Lejeune. Documented illnesses there include birth defects, infertility, miscarriages, live and kidney disorders, autoimmune diseases, cardiovascular diseases, Parkinson’s disease, Non-Hodgkin’s lymphoma, and at least nine different cancers.
What About Federal Regulation of PFAS?
When the Toxic Substances Control Act of 1976 was enacted, PFAS were not included. The Clean Water Act and the Clean Air Act of the 1970s also didn’t include PFAS. The companies who originally created them were supposed to research the potential harms of these chemicals and inform the Environmental Protection Agency (EPA) of the results. They kept the results of their research secret for decades.
The 1980 Superfund Law created a tax on petroleum and chemical industries and billions of dollars were used to clean up abandoned hazardous waste sites, but thousands of sites were not included.
In August of 2022, the EPA proposed that two commonly used PFAS should be classified as hazardous substances and there should be advisories on their presence in drinking water. If implemented, these advisories are not laws, and therefore wouldn’t carry the force of law.
What Are the States Doing About PFAS?
Rather than wait for the EPA and federal regulations to take action, many states are stepping up. They are enforcing drinking water standards, adopting PFAS guidance and notification actions, replacing PFAS in certain products with safer alternatives, and more.
- Maine and Washington state agencies have the authority to ban PFAS in many products
- Eleven states have begun removing PFAS from food packaging
- Five states are restricting PFAS in carpets, rugs and more
- California, Colorado and Maryland are eliminating PFAS from cosmetics
- Eleven states have banned the sale of firefighting foam with PFAS
- Maine became the first state to ban PFAS in all new products except those essential to health and safety that doesn’t have a PFAS-free alternative
Sixteen states have filed lawsuits against PFAS manufacturers like the 2018 lawsuit by the state of Minnesota against 3M that resulted in a settlement of $850 million. Industry advocates oppose a federal ban on PFAS and while there is ongoing disagreement among legislators on what federal regulations should be, states are taking independent action.

by manovermachine | Nov 16, 2022 | Philips Recalled BiPAP, CPAP, Ventilators
News from the FDA, DOJ and the stock market
November 16, 2022 – This week The New York Times published an article by investigative journalist Christina Jewett with some updates on the status of the Philips recall and the various lawsuits that are pending.
To review, the problems with their various breathing devices became apparent by 2015 and Philips Respironics didn’t do anything at the time. According to the Food and Drug Administration (FDA), years went by before the “company made cursory efforts to examine the problem.”
In April of 2021, the company claimed that it “realized the flaking foam contained potentially cancer-causing particles, setting off the largest and most disruptive medical device recall in more than a decade.” Since then, the recall of nearly fifteen million devices worldwide has caused patients to endure long waits for a replacement device. Some patients are receiving “refurbished” devices which they are reluctant to use.
“The F.D.A. shares the frustrations expressed by patients who are awaiting a resolution for this recall,” Dr. Jeff Shuren, director of the F.D.A.’s device center, said in a statement. “We have employed rarely used regulatory tools to hold Philips accountable and we will continue to communicate with the firm to assure they take appropriate steps to correct the product.”
The DOJ and Philips in Negotiations
The U.S. Justice Department is now negotiating the terms of a consent decree with Philips, “underscoring the deep concern about what the company knew – or should have known – before millions of people received devices that many believe caused devastating illnesses.”
Roy Jakobs, chief business leader for Philips’ Connected Care unit, told investors this past July that “We are in confidential discussions. We cannot disclose yet what the terms are and what impact they will have, but we understand that you are very much interested in that and the moment we can share we will share more details around this.”
Financial Impact and Layoffs
Fallout from the recall has resulted in the market value and stock plummeting by 70 percent and the company has been forced to lay off thousands of employees.
More than 69,000 complaints pertaining to cancer, difficulty breathing, and chest pain are being reviewed. There are 168 reported deaths, although experts say it may be difficult to determine whether the device caused any singular illness or death.
Finding and contacting patients who have these devices has been hampered by poor record-keeping and the industry-wide absence of a device tracking system.
Investigations Continue
The FDA has done extensive document reviews and has inspected the Philips Respironics facility in Murrysville, Pennsylvania. They found that by 2015, the company had learned that the foam in the devices was degrading. Emails and test reports showed that the foam could break down in as little as a year.
Philips finally began a formal internal investigation in 2019, and issued the recall in June 2021. By December 2021, their hazard assessment concluded that the device fumes presented a moderate risk for injury, rather than a serious risk. The FDA, however, considered their test methods flawed. Steve Klink, Philips spokesman, has said “At this stage, we can only apologize for the concern that has arisen, and we are working really hard to get to the bottom of the actual health risks.”

by manovermachine | Oct 18, 2022 | Camp Lejeune Contaminated Water
The DisabledVeterans.org blog reminds veterans about possible benefits offset
The Honoring our Promise to Address Comprehensive Toxics Act of 2022 (PACT Act) that the Biden administration signed into law in August 2022 does include the Camp Lejeune Justice Act. This act made it possible for veterans and affected civilians to receive compensation for injuries due to exposure to the contaminated water at Camp Lejeune between August 1, 1953 and December 31, 1987.
Prior to the PACT Act, the Federal Tort Claims Act and 38 USC § 1151 established that veterans cannot collect twice for the same injury. Therefore it is possible that an offset to compensation through the VA, Medicare, or Medicaid will apply if the medical malpractice claims against the Department of Veteran Affairs is successful. This is also true for civilians.
It’s not clear how the safeguards against “double dipping” will be applied, but the PACT Act states:
2) Health and disability benefits relating to water exposure–Any award made to an individual, or legal representative of an individual, under this section shall be offset by the amount of any disability award, payment, or benefit provided to the individual, or legal representative–
(A) under–
(i) any program under the laws administered by the Secretary of Veterans Affairs;
(ii) the Medicare program under title XVIII of the Social Security Act (42 U.S.C. 1395 et seq.); or
(iii) the Medicaid program under title XIX of the Social Security Act (42 U.S.C. 1396 et seq.); and
(B) in connection with health care or a disability relating to exposure to the water at Camp Lejeune.
PACT Act § 804(e)(2).
It’s possible that the offset provision in the PACT Act will incorporate benefits and health care already paid plus future projected benefits and health care services. If a veteran is awarded compensation through litigation, the possible PACT Act offset would not apply to any awards for pain and suffering and loss of income. However, until clear guidance comes from the VA and Social Security on how they plan to implement the offset, it’s speculation at this point.

by manovermachine | Sep 28, 2022 | 3M Earplugs Hearing Loss
Mediation session scheduled for October 3
A federal judge has ordered additional mediation sessions in the ongoing efforts to settle 3M earplug lawsuits that have been filed on behalf of more than 250,000 military veterans. The initial 3M earplug settlement talks were held on September 15 and 16, before Special Master Randi Ellis. U.S. District Judge Casey Rodgers in the Northern District of Florida, who has been presiding over the multidistrict litigation (MDL), has issued a statement saying that the mediation “was worthwhile and productive” and has ordered Ellis to schedule another session to be conducted by October 3, 2022.
Judge Rodgers is also preparing multiple tracks of claims for trial and issued a separate order, reinstating the deadline for claimants to provide their DD214 forms along with completing census forms and continuing transitioning claims from an administrative docket to an active docket. In reinstating the filing requirements, the Judge added 56 days to the initial deadline to get these materials submitted and/or transitioned.
The Combat Arms earplugs sold to the military by 3M Company and its Aearo Technologies unit proved to be defective and failed to provide adequate protection to the service members who used them between 2004 and 2015. Plaintiffs’ claims vary, and the expected payout per person will range, depending on the extent and duration of each veteran’s hearing loss. Estimates of the total cost of a 3M settlement range between $10 billion and more than $100 billion, depending on how future claims are handled.




